Anti-diabetic drug can be re-purposed as Covid treatment: Study
By IANS | Published: July 1, 2021 03:06 PM2021-07-01T15:06:07+5:302021-07-01T15:20:07+5:30
Hyderabad, July 1 Amid the rush to develop cures for the coronavirus, a study by a University of ...
Anti-diabetic drug can be re-purposed as Covid treatment: Study
Hyderabad, July 1 Amid the rush to develop cures for the coronavirus, a study by a University of Hyderabad (UoH) incubated startup, has discovered that an anti-diabetic drug has potential use as a ready to use, cost-effective solution for safely treating Covid patients.
In-vitro and in-silico studies conducted by ReaGene Innovations Private Limited, a startup company incubated at the ASPIRE-BioNEST, and INDRAS Private Limited, indicate that re-purposing the anti-diabetic drug Ertugliflozin, might provide a therapeutic solution to the Covid-19 infection.
Ertugliflozin, an FDA approved drug for type-2 diabetes, works as SGLT-2 inhibitor by removing excessive glucose through urine.
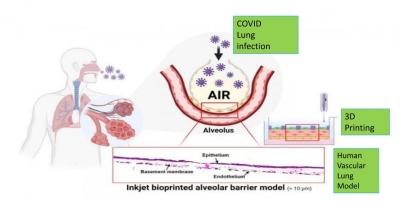
The research findings indicate that this re-purposed drug not only binds effectively to the receptor binding domain of the spike protein of Covid-19 and further blocks binding to human ACE2 but also displays significant anti-inflammatory and anti-thrombotic properties in a 3D human vascular lung model, both of which are fundamentals in Covid infection.
This is the first result that offers a safe, ready-to-use, cost-effective solution to humans who contract Covid.
"It has immense potential to treat Covid infection, and our research proves its efficacy in the test-tube assays", said Dr Uday Saxena, CEO of ReaGene Innovations, co-founded with Dr Subramanyam Vangala, and Dr Sreedhara Voleti, MD of INDRAS.
INDRAS focus is on consulting, contracting, and collaborative solutions to in-silico drug design, has prioritized about 8,000 FDA approved drugs to top-10 from their computational studies, which were further experimented by ReaGene Innovations for various in-vitro assays on cytokine storm, antithrombotic properties, and inflammatory marker reduction through various in vitro assays.
The path to find such a re-purposed drug was critically planned and completed within a year of funding from the IT giant, Tech Mahindra.
The outcomes of the results of this research were recently published in a journal (BioRxIV) and a joint patent has been filed both in India as well as internationally under Patent Cooperation Treaty.
The results obtained are highly encouraging, and further in animal models towards preclinical, and clinical outcomes in humans are yet to be conducted for this drug to be officially nominated as a therapeutic agent for COVID-19, the company said in a statement.
ASPIRE-BioNEST is a life sciences incubator jointly funded by BIRAC, a section-8 not-for-profit organization of Department of Biotechnology, Govt of India, and University of Hyderabad (UoH) for nurturing scaling technologies through entrepreneurship.
Disclaimer: This post has been auto-published from an agency feed without any modifications to the text and has not been reviewed by an editor
Open in app