Pfizer says its antiviral pill Paxlovid cuts hospitalisation rate, death risk due to COVID by 90%
By Lokmat English Desk | Published: November 6, 2021 01:08 PM2021-11-06T13:08:03+5:302021-11-06T13:08:03+5:30

American pharmaceutical and biotechnology corporation Pfizer Inc on Friday said its experimental antiviral COVID pill cuts rates of hospitalization and death by nearly 90% in high-risk adults, as the drugmaker joins the race to bring the first easy-to-use medication against the coronavirus to the US market.
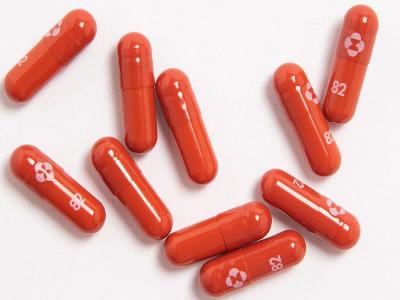
“The investigational novel COVI-19 oral antiviral candidate PAXLOVID was found to reduce the risk of hospitalization or death by 89% compared to placebo in non-hospitalized high-risk adults with COVID-19,” Pfizer said.

Issuing a statement, Pfizer said it will ask the FDA and international regulators to authorize its pill as soon as possible, after independent experts recommended halting the company’s study based on the strength of its results.

Once Pfizer applies, the FDA could make a decision within weeks or months.
The statement from Pfizer comes at a time when the researchers across the globe are racing to find a pill against COVID-19 that can be taken at home to ease symptoms, speed recovery and reduce the crushing burden on hospitals and doctors.

On Friday, Pfizer released preliminary results of its study of 775 adults and said the patients taking the company’s drug along with another antiviral had an 89% reduction in their combined rate of hospitalization or death after a month, compared to patients taking a dummy pill.
Fewer than 1% of patients taking the drug needed to be hospitalized and no one died. In the comparison group, 7% were hospitalized and there were seven deaths.

“We were hoping that we had something extraordinary, but it’s rare that you see great drugs come through with almost 90% efficacy and 100% protection for death, said Dr. Mikael Dolsten, Pfizer’s chief scientific officer, in an interview.
Study participants were unvaccinated, with mild-to-moderate COVID-19, and were considered high risk for hospitalization due to health problems like obesity, diabetes or heart disease. Treatment began within three to five days of initial symptoms, and lasted for five days.

Pfizer reported few details on side effects but said rates of problems were similar between the groups at about 20%. An independent group of medical experts monitoring the trial recommended stopping it early, standard procedure when interim results show such a clear benefit. The data have not yet been published for outside review, the normal process for vetting new medical research.