India's Covid-19 Vaccination : What are the Dos, Dont's and Possible Side Effects
By Lokmat English Desk | Published: January 15, 2021 03:29 PM2021-01-15T15:29:35+5:302021-01-15T15:39:48+5:30

Covid-19 vaccination is allowed only for 18 years and above.

Pregnant women or who are not sure of their pregnancy and lactating mothers shouldn't receive the vaccine.

Administration of Covid vaccine should be done separated by an interval of 14 days.
The second dose should be of the same vaccine of which the first dose was administered.
Interchanging Covid-19 vaccines is not allowed.

The Health Ministry cautioned about mild side effects following vaccination for both the vaccines. In case of Covishied, some mild adverse effects may occur like injection site tenderness, injection site pain, headache, fatigue, myalgia, malaise, pyrexia, chills and arthralgia and nausea.

Some mild adverse effects in case of Covaxin include injection site pain, headache, fatigue, fever, body ache, abdominal pain, nausea and vomiting, dizziness-giddiness, tremor, sweating, cold, cough and injection site swelling.
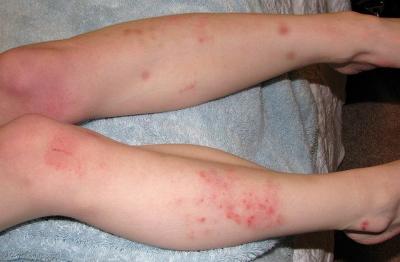
The vaccine should be administered with caution in persons with a history of any bleeding or coagulation disorder (like clotting factor deficiency, coagulopathy or platelet disorder.
Following conditions are not contraindicated for COVID-19 vaccines -- persons with past history of SARS-CoV-2 infection and or RT-PCR positive illness, history of chronic diseases and morbidities and immuno-deficiency, HIV, patients on immune suppression due to any condition.
A dose of Covishield and CoVaxin may cost in the range of ₹200 to 295 in India. The full initial procurement amount of 1.65 crore doses of COVISHIELD and COVAXIN vaccines have been allocated to all states and Union Territories in the proportion of Health Care Workers database, the Health Ministry has said.
As per the Health Ministry’s letter, in case of persons having active symptoms of SARS-CoV-2 infection, coronavirus infected patients who have been given anti-SARS-CoV-2 monoclonal antibodies or convalescent plasma and acutely unwell and hospitalised patients due to any illness, the COVID-19 vaccination is to be deferred for four to eight weeks after recovery.

The ministry cautioned against administration of the vaccine in persons with a history of anaphylactic or allergic reaction to a previous dose of COVID-19 vaccine, and in those with immediate or delayed onset anaphylaxis or allergic reaction to vaccines or injectable therapies, pharmaceutical products, food items, among others.