Cuba approves emergency use of domestic Covid vax
By IANS | Published: July 10, 2021 09:27 AM2021-07-10T09:27:04+5:302021-07-10T09:45:30+5:30
Havana, July 10 The Cuban Centre for State Control of Medicines, Equipment and Medical Devices has approved the ...
Cuba approves emergency use of domestic Covid vax
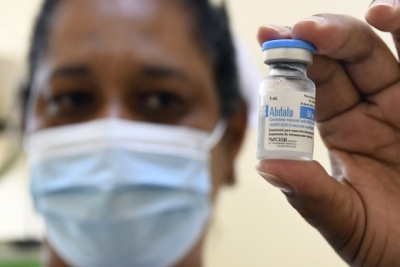
Havana, July 10 The Cuban Centre for State Control of Medicines, Equipment and Medical Devices has approved the emergency use authorisation to Abdala, one of five domestic vaccines against Covid-19.
The regulatory body said in a statement on Friday that it granted the emergency use authorization to Abdala "once it was confirmed that the requirements and parameters demanded in terms of quality, safety and efficacy were met", Xinhua news agency reported.
According to Cuban scientists, the vaccine developed by the Center for Genetic Engineering and Biotechnology (CIGB) has an efficacy rate of 92.28 per cent in three doses administered at 14-day intervals.
The decision makes Abdala the first vaccine made in Latin America that meets the requirements for large-scale use in the country and even for export.
According to the latest data from the Cuban Ministry of Health, more than 7 million doses of domestic vaccines have been administered, and just over 3 million people have received at least one dose as participants in clinical trials or in emergency intervention.
Cuba on Friday saw its worst pandemic figures since the start of the outbreak 16 months ago.
With 6,422 new cases and 28 more fatalities, the overall infection tally and death toll increased to 224,818 and 1,459.
Disclaimer: This post has been auto-published from an agency feed without any modifications to the text and has not been reviewed by an editor
Open in app