Pakistan begins Phase 3 trails of their Coronavirus vaccine
By Lokmat English Desk | Published: August 19, 2020 12:58 PM2020-08-19T12:58:01+5:302020-08-19T12:58:01+5:30

Drug Regulatory Authority of Pakistan has approved the clinical trials for an indigenously developed COVID-19 vaccine. As per Dawn reports, the committee has recommended that the trial be held in Indus Hospital Karachi.

The trials will be carried out by Pakistan's International Centre for Chemical and Biological Sciences, Karachi, in collaboration with a Chinese firm.
The state-run National Institute of Health (NIH) said on August 17 that the Phase III clinical trial would be the first of its kind in Pakistan.
"This will be the first ever Phase III clinical trial for any vaccine in Pakistan," the NIH said in a statement.
Pakistan's inclusion in the large-scale testing on humans would help it secure "preferential vaccine supply and pricing,” it added.
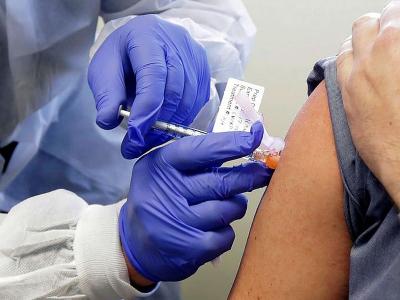
The experimental vaccine is already undergoing Phase III trials in China, Russia, Chile, and Argentina. Mexico and Saudi Arabia are also planning to join the clinical trials. The trial will be conducted at several medical facilities across Pakistan.

The experimental vaccine is already undergoing Phase III trials in China, Russia, Chile, and Argentina. Mexico and Saudi Arabia are also planning to join the clinical trials.COVID-19 has claimed more than 6,000 lives in Pakistan and infected some 290,000, according to official numbers.
More than 150 vaccines are being developed and tested around the world to stop the COVID-19 pandemic, with 25 in human clinical trials, according to the World Health Organization.

The race for a vaccine has set off a global competition among countries trying to ensure they gain timely, secure, and affordable access to any vaccine or treatment.
पाकिस्तानात आतापर्यंत तब्बल 2,89,215 जणांहून अधिक लोकांना कोरोनाची लागण झाली आहे.
As many as 200 volunteers from Karachi, representing various ethnic groups, have been registered. The trial will be completed in 56 days during which three injections of inactivated virus will be administered to the volunteers both male and female.
The document said the National Data Safety Monitoring Committee will closely monitor the safety of the patients and submit reports on a monthly basis.
Pakistan’s coronavirus cases reached 2,89,832 on Tuesday after 617 new infections were diagnosed in the last 24 hours. Fifteen more people died during this period, taking the number of COVID-19 deaths to 6,190 across the country, the health ministry said on Tuesday.
Minister for Planning Asad Umar, who also heads the National Coordination and Operation Centre on COVID-19, said he was hopeful that the trials on the vaccine would be successful.