Drug Controller General approves clinical trial of convalescent plasma in COVID-19 patients
By Lokmat English Desk | Published: April 18, 2020 01:52 PM2020-04-18T13:52:15+5:302020-04-18T13:52:41+5:30
In what can be termed as a major development the central drug regulator has given its go-ahead to a ...
Drug Controller General approves clinical trial of convalescent plasma in COVID-19 patients
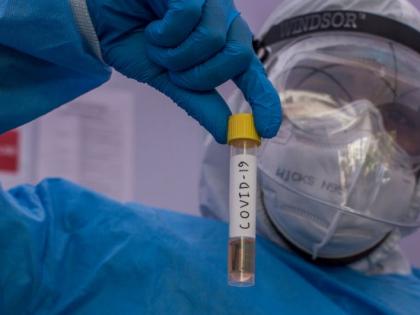
In what can be termed as a major development the central drug regulator has given its go-ahead to a proposdal by the Indian Council of Medical Research for the clinical trial of convalescent plasma in COVID-19 patients, as per the protocol developed by ICMR. The Drug Controller General of India said ICMR has submitted a list of institutes, which have shown an interest in the trial, to the Central Drugs Standard Control Organisation and they may do so in consultation with the health research body.
"It is to inform that in light of public interest the proposal of ICMR for conducting the said trial has been reviewed through the Subject Expert Committee in its meeting held on April 13 under accelerated approval process in light of the current prevailing situation of COVID-19 and based on the recommendation of the committee."The CDSCO has conveyed its no objection for conduct of the clinical trial subject to certain amendments in the protocol and various conditions under the Drugs and Clinical Trial Rules 2019," the central drug regulator said in a notice.In convalescent plasma therapy, antibodies from the blood of patients who have recovered from COVID-19 are used to treat severely infected patients. The number of cases reported in India crosses the 14,000, including 11,906 active cases and 480 deaths, according to the health ministry.
Open in app