Favipiravir and Remdesivir get approval for COVID-19 treatment
By Lokmat English Desk | Published: June 20, 2020 10:15 AM2020-06-20T10:15:24+5:302020-06-20T10:19:40+5:30

Favipiravir and Umifenovir (Arbidol) are the drugs approved by Drug Controller General of India (DCGI) for clinical trials to treat Covid-19, at Council of Scientific and Industrial Research (CSIR).
Dr Shekhar Mande, Director General, CSIR, said the regulatory body had approved Favipiravir for the second phase of clinical trials, and multicentre trials were underway across different locations, including Mumbai and Pune.
Along with Favipiravir are other drugs – ACQH, Umifenovir (Arbidol) and Mycobacterium W (anti-leprosy drug), which are now in clinical trials.

The focus is on repurposed drugs and with 10 in the basket so far, Dr Mande said he was hopeful of results in the next two to three months.

Favipiravir is an antiviral drug for emergency use against Covid-19. Scientists at CSIR were banking on data from clinical trials in Russia, where the FDA has approved the drug and their own partial data. “The drug has been approved for Phase II clinical trials,” Dr Mande said.
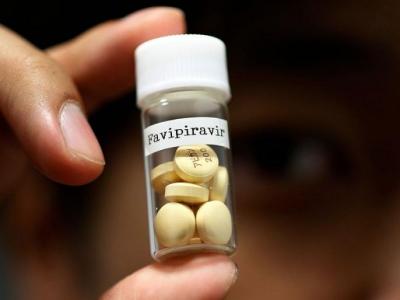
Favipiravir is also being used to treat new influenza viruses in Japan. It is the generic version of the drug Avifavir, originally sold in Japan under the brand name Avigan.

This is a pyrazine carboxamide derivative and is studied to treat other viral infections. One of the repurposed drugs, Avifavir could shorten recovery time for patients with coronavirus.

Multicentre clinical trials of phytopharmaceutical ACQH have also begun. It is a plant extract found in the tribal belts of Gujarat, Jharkhand and Madhya Pradesh.

Another drug Mycobacterium W (Mw) has been under clinical trials and Dr Mande said he was hopeful of results in the next 10 days.

There are some positive indications from the government-run AIIMS in Bhopal, which has been conducting the drug trials for the treatment of Covid-19.

Mw was used in the treatment of leprosy and CSIR has tied up with Cadila Pharmaceuticals to check for its efficacy in treating Covid-19.

The Umifenovir drug also has a good safety profile and acts by preventing entry of viruses into human cells.

It also works to prime the immune system and is used for the treatment of influenza. Clinical trials to evaluate its efficacy are also underway, Dr Mande said.
While the trials are still ongoing, the DCGI approved the drug as the interim results so far have been encouraging, the source said.

The DCGI had recommended that the drug should be used only in adults and not on patients with severe liver and renal impairment and pregnant and lactating women, the source said, adding it should be used with caution in patients having a history of abnormalities in uric acid metabolism or gout.























